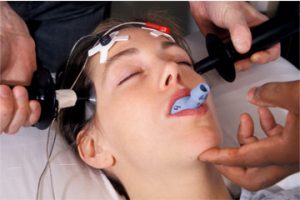
The U.S. Food and Drug Administration (FDA) approved on April 19, 2019 a medical device for so-called attention deficit hyperactivity disorder (ADHD). The prescription-only device, called the Monarch external Trigeminal Nerve Stimulation (eTNS) System from NeuroSigma, is for patients ages 7 to 12 years old who are not currently taking prescription ADHD drugs.
This device delivers an electric current to the brain (through the V1 branch of the 5th cranial nerve) with an electrode taped to the forehead. It costs about $900 to start, with additional costs for more of the electrode patches which are only used once each. It is not currently reimbursed by insurance. READ MORE…
VLA Comment: Well here we are Humanity…little lab rats! Poison them kids, dogs and
plants too (vaccines, gmo food, geoengineering, fluoride, pesticides,
herbicides) them-disable them-electrocute them…and the new infanticide
bills…kill them immediately)*