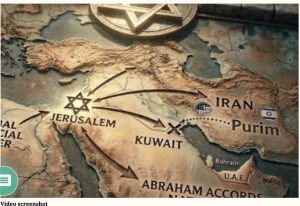
VLA COMMENT: THE REAL PLAN:
GREATER ISRAEL TOTAKE OVER ALL THE TRADE CHOKEPOINTS OF WEST ASIA including building a canal parallel to the Suez Canal…The Ben Gurion Canal. Including taking over the Iran Port (Strait of Hormuz).
MUST WATCH PEPI ESCOBAR TAKES US THROUGH THE IRANIAN PORT THAT ISRAEL COVETS
In case it is blocked: https://www.youtube.com/watch?v=AgsStCtRSWE
WHAT THIS IRAN TAKE OVER IS REALLY ABOUT
VLA Comment: The Golden Corridor is the chokepoint of West Asia (formally known as the middle east).This corridor is what we (Israel & USA) want so we and the master race of Greater Israel can control global trade of West Asia. It runs through Iran. It belongs to Iran. It is a huge global channel that when finally constructed commands 40% of oil distribution globally and more commodities.